Reproducibility of a Digital Twin of Glimepiride for Personalized and Stratified Diabetes Treatment
Abstract¶
A digital twin in the form of a whole-body physiologically based pharmacokinetic (PBPK) model of glimepiride was developed to systematically evaluate the influence of patient-specific factors on drug disposition. Based on curated data from 20 clinical studies, the model simulates the absorption, distribution, metabolism and excretion (ADME) of the drug while accounting for variability in renal and hepatic function, CYP2C9 genetic variants and bodyweight. The model is implemented in the Systems Biology Markup Language (SBML) standard and simulations are performed using scripts that utilise the libRoadRunner library to run simulations and generate results. Here, we demonstrate the computational reproducibility of the key findings from the primary publication, thereby verifying the consistency and reproducibility of the model implementation with the published results.
1Introduction¶
Glimepiride is a second-generation sulfonylurea whose pharmacokinetics are subject to pronounced inter-individual variability McCall, 2001Langtry & Balfour, 1998. The disposition of the drug is governed by its extensive metabolism in the liver, primarily by the cytochrome P450 2C9 (CYP2C9) enzyme, into its major metabolites M1 (partially active) and M2 (inactive) Yoo et al., 2011Suzuki et al., 2006. This metabolic pathway is a key determinant of the drug’s safety and efficacy profile Douros et al., 2017.
In the primary publication Elias & König, 2025, a whole-body physiologically based pharmacokinetic (PBPK) model was developed to mechanistically integrate the key factors driving this variability. The model accounts for genetic polymorphisms in CYP2C9 Yoo et al., 2011Suzuki et al., 2006, as well as patient-specific renal function, hepatic function, and bodyweight Rosenkranz, 1996Rosenkranz et al., 1996Shukla et al., 2004. The model’s structure and parameters were derived from a comprehensive dataset that was curated from 20 published clinical studies which is available via the pharmacokinetics database PK-DB Grzegorzewski et al., 2021. The model’s development and scientific validation are described in detail in the primary paper.
Here, we present the original model, encoded in the Systems Biology Markup Language (SBML) Hucka et al., 2019Keating et al., 2020, and the accompanying simulation scripts required to run the simulations and reproduce the key results presented in the primary publication.
2Model Description¶
The disposition of glimepiride is described using a whole-body physiologically based pharmacokinetic (PBPK) model. The model consists of interconnected compartments that simulate key organs involved in the drug’s absorption, distribution, metabolism, and excretion (ADME). The mathematical framework of the model are ordinary differential equations (ODE). A schematic overview of the model structure is provided in Figure 1.
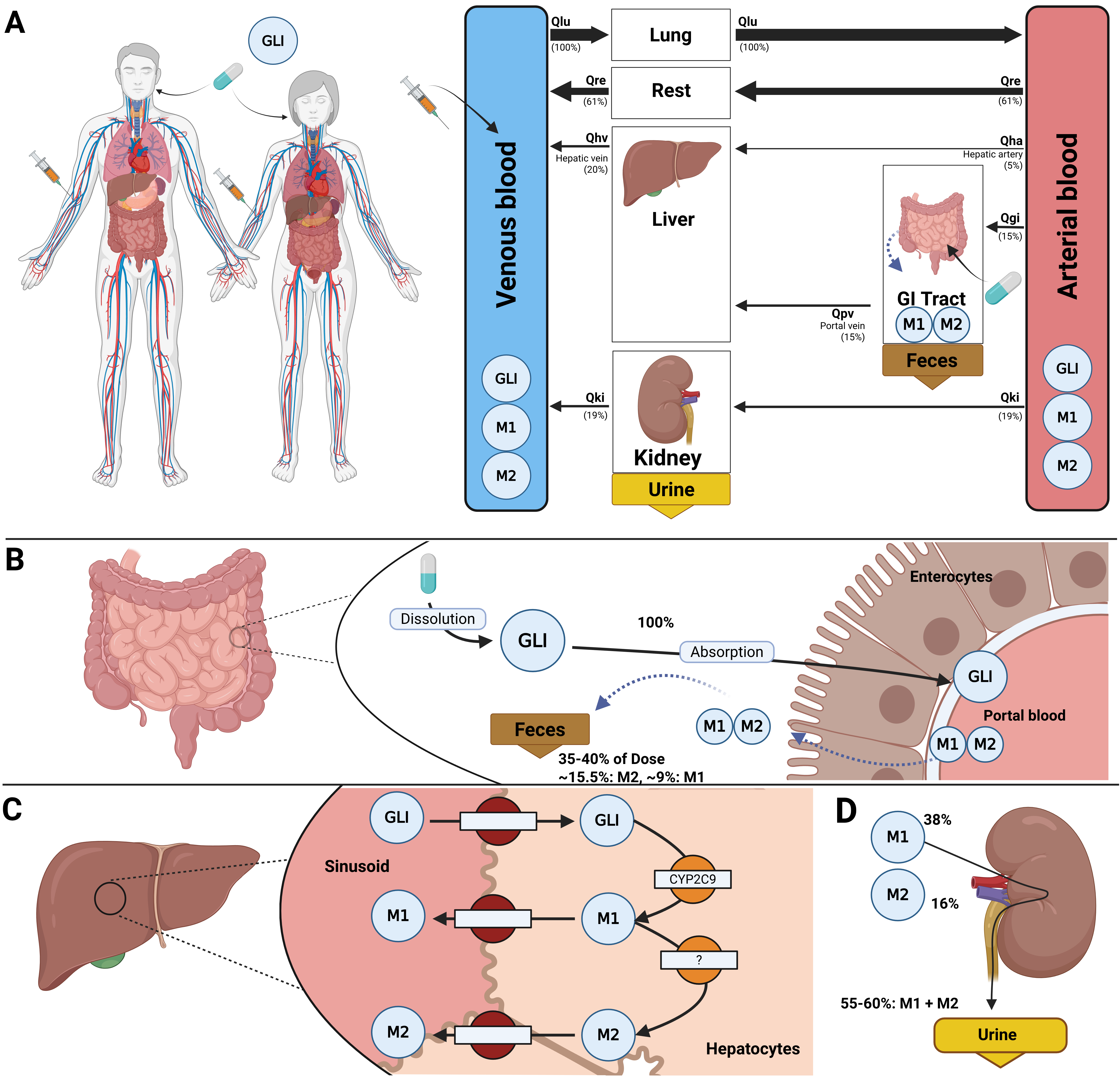
Figure 1:Whole-body PBPK model of glimepiride. A) Whole-body model illustrating glimepiride (GLI) administration (oral and intravenous), its systemic circulation via venous and arterial blood, and the key organs (liver, kidney, GI tract) involved in GLI metabolism, distribution, and excretion. B) Intestinal model showing dissolution and absorption of GLI by enterocytes. No enterohepatic circulation of M1 and M2 is assumed, but reverse transport via enterocytes is included. C) Hepatic model depicting CYP2C9-mediated metabolism of GLI to M1 and M2. D) Renal model highlighting the elimination of M1 and M2 via urine; unchanged GLI is not excreted renally.
The model integrates the three main organ submodels which are interconnected via the systemic circulation. The gastrointestinal tract model simulates the dissolution of orally administered glimepiride, its subsequent first-order absorption, and the fecal excretion of metabolites. The liver model describes the primary metabolic pathway, where glimepiride is converted to its metabolites, M1 and M2. This biotransformation is modelled using Michaelis-Menten kinetics. The kidney model implements the renal excretion of the M1 and M2 metabolites from the plasma into urine via first-order clearance processes.
The model accounts for patient-specific factors through the corresponding scaling parameters. Genetic variability in CYP2C9 activity is controlled by the fcyp2c9 parameter, which modulates the catalytic activity of the conversion of glimepiride via its maximal metabolic rate. The effect of renal impairment is implemented via the frenal function parameter, which directly scales the renal excretion rates of the metabolites. Hepatic impairment is simulated using the fcirrhosis parameter, which modifies functional liver volume and liver blood flow. The influence of bodyweight is incorporated through allometric scaling of all relevant organ volumes and blood flows.
The PBPK model and its tissue-specific submodels were developed using the Systems Biology Markup Language (SBML) Hucka et al., 2019Keating et al., 2020. Programming and visualization of the models were performed using the sbmlutils König, 2024 and cy3sbml König et al., 2012 libraries. Numerical solutions for the ordinary differential equations (ODEs) underlying the model were computed using sbmlsim König, 2021, which is powered by the high-performance SBML simulation engine libRoadRunner Welsh et al., 2023Somogyi et al., 2015. The tissue submodels were developed as SBML submodels and coupled with the whole-body model using the hierarchical model composition (comp) SBML extension Smith et al., 2015. The complete model, submodels, reference simulations and visualisations are available as a COMBINE archive (OMEX) Bergmann et al., 2014Bergmann et al., 2015. The model is annotated with extensive metadata using the open modelling and exchange (OMEX) metadata specification Neal et al., 2020Neal et al., 2019. The model was validated using the SBML validator, with the model passing all validation tests including unit tests without errors or warnings. The FAIRness of the model was increased by following the FAIRification of computational models in biology workflow Balaur et al., 2025.
The model and all associated materials (mathematical formulation, simulation scripts, parameters, and documentation) are publicly available in SBML format and OMEX archive under a CC-BY 4.0 license at https://
For interactive exploration of the model, a web application was developed. It is publicly accessible at https://
3Computational Simulation¶
All simulations were performed using Python 3.13 together with the high-performance libRoadRunner simulation engine. The workflow was tested across multiple platforms, including Ubuntu 24.04/25.04 and Windows 11. For SBML model handling and simulation we relied on the sbmlutils and sbmlsim libraries, while data management and figure generation were carried out with standard scientific Python packages.
To ensure reproducibility, we provide two equivalent setups for regenerating all analyses and figures presented in Section 4: (1) a local Python installation using uv, and (2) a containerized workflow using Docker. Both approaches reproduce all results from the primary publication. Reproducibility is continuously validated through automated integration tests, with results available at https://
3.1Python with uv (local install)¶
This workflow installs the package directly on your machine using uv.
Prerequisite: uv must be installed on your system (https://
Clone the repository and move into its folder:
git clone https://github.com/matthiaskoenig/glimepiride-model.git
cd glimepiride-modelSet up the uv virtual environment and install all dependencies:
uv venv
uv syncRun the full analysis:
uv run run_glimepiride -a all -r resultsAll reproduced figures and outputs are written to ./results/ inside the repository.
Alternatively you can use any other way to setup a local python environment (e.g. conda) and install the package after cloning the repository via
pip install -e . or directly from the main branch via
pip install git+https://github.com/matthiaskoenig/glimepiride-model.git@mainThe full analysis can be run in the python environment via
(env) run_glimepiride -a all -r results3.2Docker (containerized)¶
This workflow runs the analysis in a preconfigured Docker container.
Prerequisite: Docker must be installed on your system (https://
Start the container and mount a local results/ directory:
docker run -v "${PWD}/results:/results" -it matthiaskoenig/glimepiride:latest /bin/bashInside the container, run the analysis. Results will be written to the mounted folder:
uv run run_glimepiride -a all -r /resultsThe reproduced figures and outputs are then accessible on the host system in ./results/.
If file access is restricted on Linux due to permissions, adjust ownership and rights as follows:
sudo chown $(id -u):$(id -g) -R "${PWD}/results"
sudo chmod 775 "${PWD}/results"3.3Available Options¶
Specific parts of the analysis can be executed by providing command-line arguments. A full overview of the available options is obtained via:
uv run run_glimepiride --help3.4Outputs¶
The workflow reproduces all figures and results from the primary publication, including:
Clinical study reproductions (Figure 2)
Supplementary simulations (Figure 3)
Additional pharmacokinetic analyses (Figure 4)
All results are stored in the results/ directory. This directory contains the individual figure panels in PNG format as well as an automatically generated HTML report (index.html) that consolidates all figures into a single document. The content of this report directly corresponds to Figures 2–4 in the manuscript.
4Reproducibility Goals¶
The reproducibility of the glimepiride PBPK model was confirmed by reproducing key figures from the original publication and its supplementary material. The figures presented here are a selection chosen to demonstrate consistent reproduction of results across different dose levels, pathophysiological states and genotypes. Table 1 and Table 2 provide an overview of the simulation observables and the parameter changes specific to each study or experiment. The model and simulation scripts can be used to reproduce the full set of results from the original study and its supplements, including the parameter scan (pharmacokinetic parameter plots), as these are based on the results of the time-course profiles.
Table 1:Plotted observables and parameter changes per study.
| StudyID | Plotted (sid) | Changes |
| Ahmed2016 | Cve_gli | BW = 75 kg PODOSE_gli = 1 mg |
| Badian1994 | Cve_gli, Cve_m1, Cve_m2, Cve_m1_m2, Aurine_m1, Aurine_m2, Aurine_m1_m2 | BW = 78 kg PODOSE_gli = 1 mg Ri_gli = 1 mg/min (IV start) |
| Badian1996 | Cve_m1, Cve_m2, Aurine_m1, Aurine_m2, Aurine_m1_m2 | BW = 75 kg Ri_m1 = 1.5 mg/min (IV start) |
| Choi2014 | Cve_gli, Cve_m1 | BW = 70.8 kg PODOSE_gli = 4 mg |
| FDA1995 | Cve_gli, Cve_m1, Cve_m2, Cve_m1_m2, Aurine_m1_m2, Afeces_gli, Afeces_m1, Afeces_m2, Afeces_m1_m2 | PODOSE_gli = 1 mg Ri_gli = 1 mg/min (IV start) |
| Helmy2013 | Cve_gli | BW = 72.75 kg PODOSE_gli = 1, 2, 3, 4, 6 mg |
| Kasichayanula2011c | Cve_gli | PODOSE_gli = 4 mg |
| Kim2017 | Cve_gli | BW = 69.6 kg PODOSE_gli = 4 mg |
| Lee2012 | Cve_gli | BW = 71.8 kg PODOSE_gli = 2 mg LI__f_cyp2c9 = *1/*1, *1/*3, *3/*3 |
| Lehr1990 | Cve_gli, Cve_m1, Cve_m2, Cve_m1_m2 | PODOSE_gli = 3 mg |
| Liu2010 | Cve_gli, Cve_m1 | BW = 64 kg PODOSE_gli = 2 mg |
| Malerczyk1994 | Cve_gli, Aurine_m1_m2 | BW = 78 kg PODOSE_gli = 1, 2, 4, 8 mg |
| Matsuki2007 | Cve_gli | PODOSE_gli = 2 mg (single), 1 mg (multiple) |
| Niemi2002 | Cve_gli | BW = 68 kg PODOSE_gli = 0.5 mg LI__f_cyp2c9 = *1/*1, *1/*2, *1/*3 |
| Ratheiser1993 | Cve_gli, Aurine_m1_m2 | Ri_gli = 0.25, 0.5, 0.75, 1, 1.25, 1.5 mg/min (IV start) |
| Rosenkranz1996a | Cve_gli, Cve_m1, Cve_m2, Cve_m1_m2, Aurine_m1_m2 | PODOSE_gli = 3 mg KI__f_renal_function = normal, mild, moderate, severe |
| Shukla2004 | Cve_gli, Cve_m1, Cve_m2, Cve_m1_m2, Aurine_m1, Aurine_m2, Aurine_m1_m2 | BW = 72 kg (normal), 130 kg (obese) PODOSE_gli = 8 mg |
| Suzuki2006 | Cve_gli | PODOSE_gli = 1 mg LI__f_cyp2c9 = *1/*1, *1/*3 |
| Wang2005 | Cve_gli | PODOSE_gli = 4 mg LI__f_cyp2c9 = *1/*1, *1/*3, *3/*3 |
| Yoo2011 | Cve_gli | BW = 67.6 kg PODOSE_gli = 2 mg LI__f_cyp2c9 = *1/*1, *1/*3 |
Table 2:Plotted observables and parameter changes per experiment.
| StudyID | Plotted (sid) | Changes |
| BodyweightExperiment | Cve_gli, Cve_m1, Cve_m2, Aurine_m1_m2 | PODOSE_gli = 8 mg BW = 45, 70, 95, 120, 145, 170 kg |
| DoseDependencyExperiment | Cve_gli, Cve_m1, Cve_m2, Aurine_m1_m2 | PODOSE_gli = 0, 1, 2, 3, 4, 5, 6, 7, 8 mg |
| GeneticVariantExperiment | Cve_gli, Cve_m1, Cve_m2, Aurine_m1_m2 | PODOSE_gli = 4 mg LI__f_cyp2c9 = *1/*1 (1.00), *1/*2 (0.84), *1/*3 (0.62), *3/*3 (0.23) |
| RenalImpairmentExperiment | Cve_gli, Cve_m1, Cve_m2, Aurine_m1_m2 | PODOSE_gli = 3 mg KI__f_renal_function = Normal (1.00), Mild (0.50), Moderate (0.35), Severe (0.20) |
| HepaticImpairmentExperiment | Cve_gli, Cve_m1, Cve_m2, Aurine_m1_m2 | PODOSE_gli = 3 mg f_cirrhosis = Control (0.00), Mild (0.399), Moderate (0.698), Severe (0.813) |
4.1Reproduction of Key Clinical Study Simulations¶
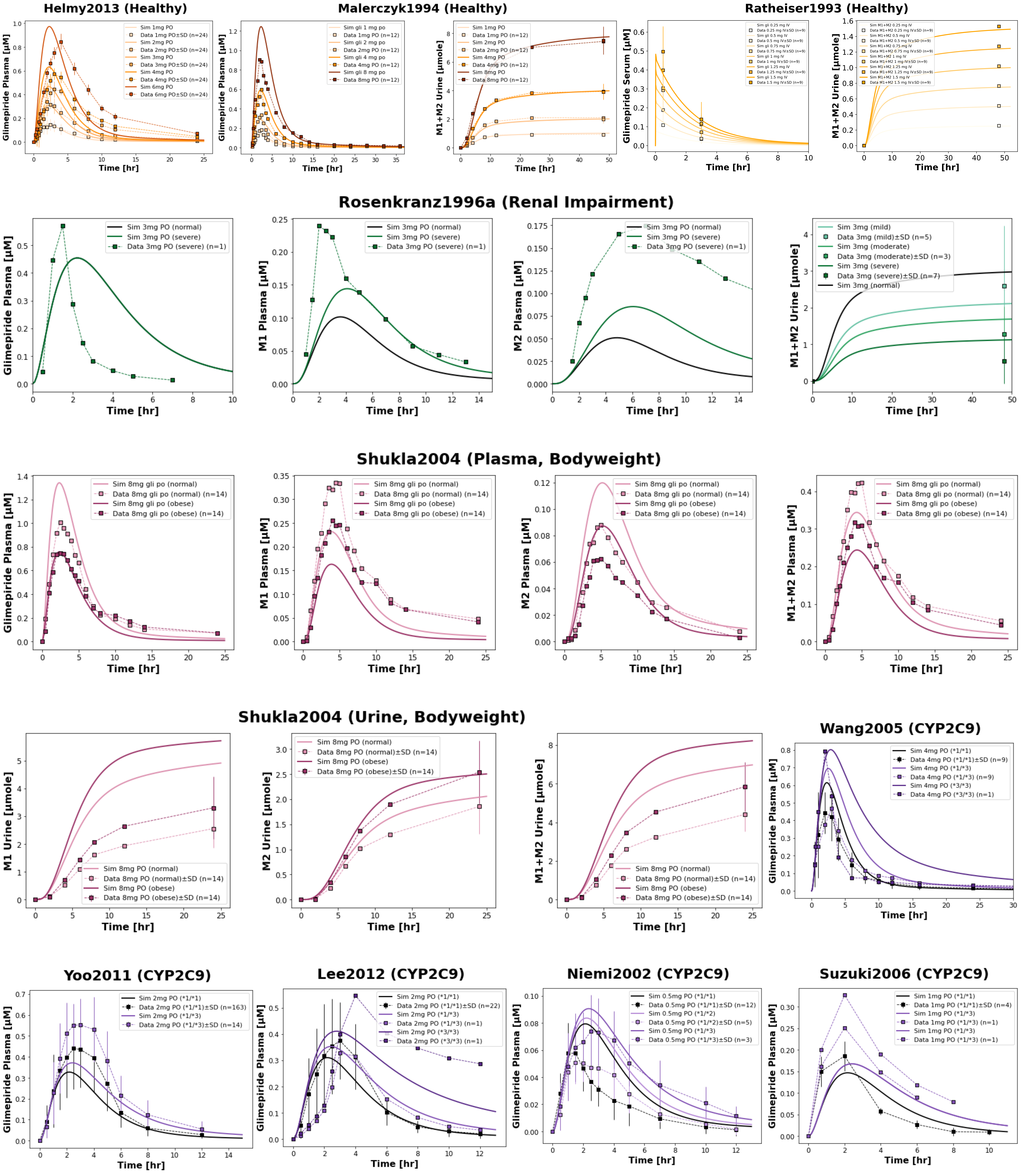
Figure 2:Reproduction of key clinical studies from the primary publication. The model successfully reproduces the influence of renal impairment (Rosenkranz1996a Rosenkranz et al., 1996), bodyweight (Shukla2004 Shukla et al., 2004), CYP2C9 genetic variants (Wang2005 Wang et al., 2005, Yoo2011 Yoo et al., 2011, Lee2012 Lee et al., 2012, Niemi2002 Niemi et al., 2002, Suzuki2006 Suzuki et al., 2006), and dose dependency (Helmy2013 Helmy et al., 2013, Malerczyk1994 Malerczyk et al., 1994, Ratheiser1993 Ratheiser et al., 1993) on glimepiride and metabolite pharmacokinetics. Simulated profiles (lines) are compared with observed clinical data (squares connected by dashed lines, with SD shown where available).
4.2Reproduction of Supplementary Clinical Study Simulations¶
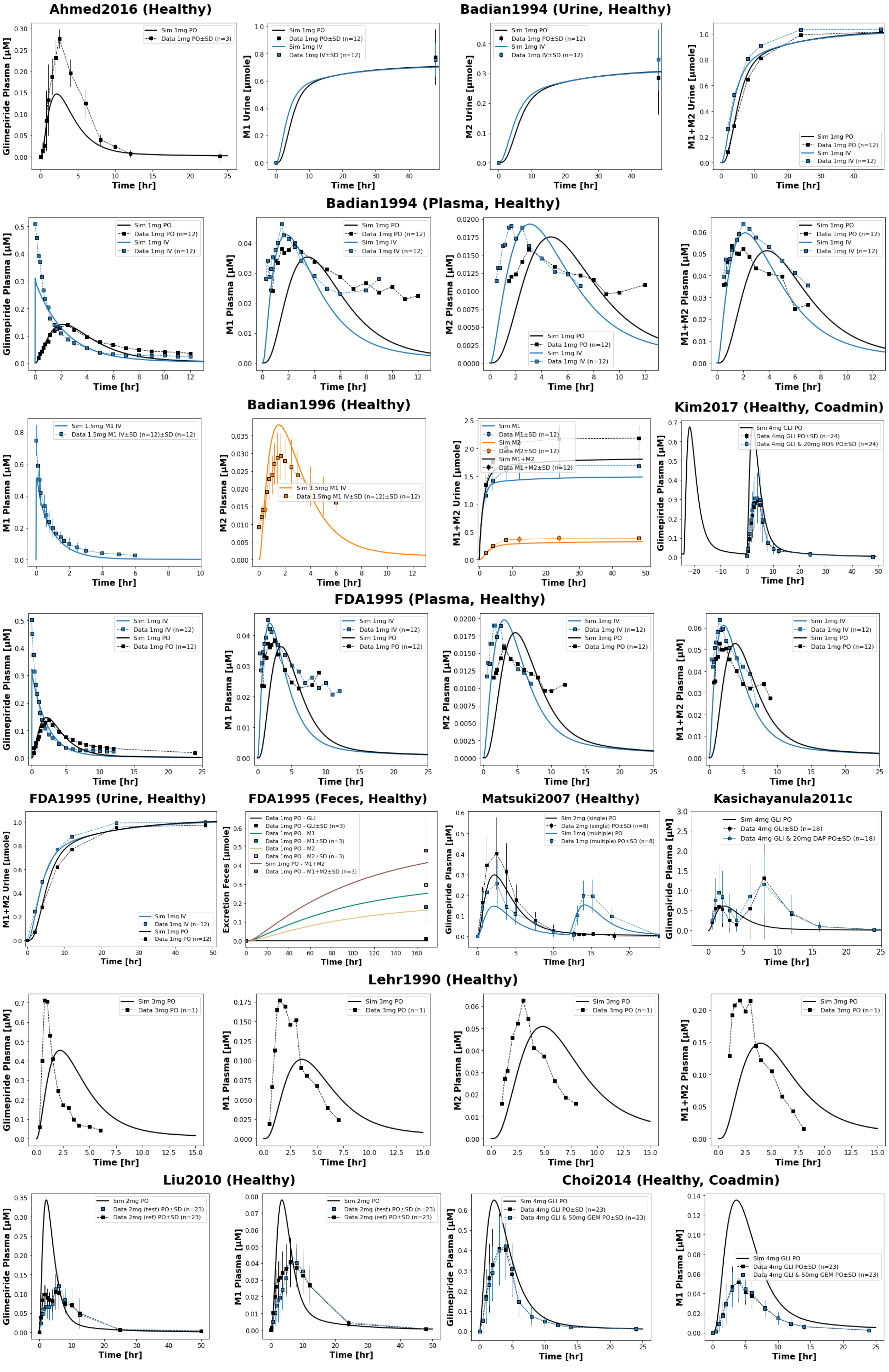
Figure 3:Reproduction of clinical study simulations from the primary publication’s supplementary material. The model accurately reproduces pharmacokinetic profiles across various study scenarios (Ahmed2016 Ahmed et al., 2016, Badian1994 Badian et al., 1994, Badian1996 Badian et al., 1996, Choi2014 Choi et al., 2014, FDA1995 U.S. Food and Drug Administration (FDA), 1995, Kasichayanula2011c Kasichayanula et al., 2011, Kim2017 Kim et al., 2017, Lehr1990 Lehr & Damm, 1990, Liu2010 Liu et al., 2010, Matsuki2007 Matsuki et al., 2007). Simulated profiles (lines) are compared with observed clinical data squares connected by dashed lines, with SD shown where available).
4.3Reproduction of Additional Simulations¶
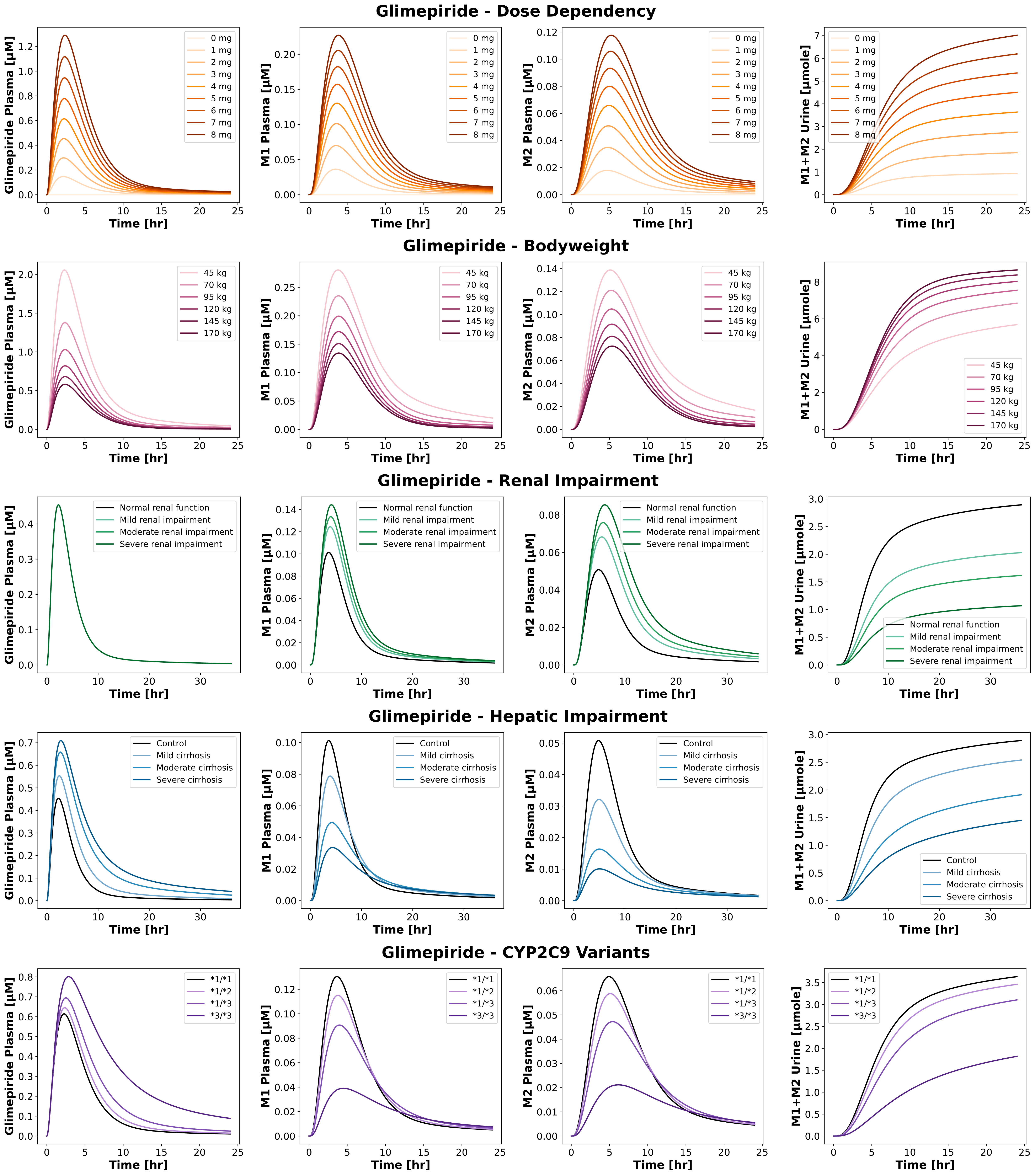
Figure 4:Reproduction of simulations from the primary publication and its supplementary material. The model accurately reproduces pharmacokinetic profiles in various scenarios, including dose dependency, bodyweight dependence, renal impairment, hepatic impairment and CYP2C9 polymorphisms.
5Discussion¶
We have demonstrated the computational reproducibility of the key findings from the glimepiride PBPK model presented in the primary publication. Using the provided simulation scripts, all figures were regenerated without modifying parameters or structure, verifying the consistency of the model. Reproducibility was confirmed across different operating systems using both a local installation with uv and a Dockerized workflow. The uv-based approach allows users to install the package and dependencies natively, while the containerized workflow provides a fully preconfigured environment and ensures consistent results independent of the local setup. Encoding the model in SBML with hierarchical composition removes ambiguity and allows modular reuse of the tissue submodels. Together with the use of community standards and FAIR practices, this provides a transparent and reusable resource that can be applied or extended in future pharmacokinetic modelling work.
Author Contributions¶
M.E. and M.K. contributed to conceptualization, methodology, data curation, development of the PBPK model, analyses, web application, and reproducibility of the computational workflow. M.E. wrote the original draft. M.K. provided supervision throughout the project and contributed to manuscript review and editing. Both authors approved the final manuscript.
Funding¶
Matthias König (MK) was supported by the Federal Ministry of Education and Research (BMBF, Germany) within ATLAS by grant number 031L0304B and by the German Research Foundation (DFG) within the Research Unit Program FOR 5151 "QuaLiPerF (Quantifying Liver Perfusion-Function Relationship in Complex Resection - A Systems Medicine Approach)" by grant number 436883643 and by grant number 465194077 (Priority Programme SPP 2311, Subproject SimLivA). This work was supported by the BMBF-funded de.NBI Cloud within the German Network for Bioinformatics Infrastructure (de.NBI) (031A537B, 031A533A, 031A538A, 031A533B, 031A535A, 031A537C, 031A534A, 031A532B).
Acknowledgments¶
Figures were created in BioRender. König, M. (2025) https://
- McCall, A. L. (2001). Clinical Review of Glimepiride. Expert Opinion on Pharmacotherapy, 2(4), 699–713. 10.1517/14656566.2.4.699
- Langtry, H. D., & Balfour, J. A. (1998). Glimepiride. A Review of Its Use in the Management of Type 2 Diabetes Mellitus. Drugs, 55(4), 563–584. 10.2165/00003495-199855040-00007
- Yoo, H.-D., Kim, M.-S., Cho, H.-Y., & Lee, Y.-B. (2011). Population Pharmacokinetic Analysis of Glimepiride with CYP2C9 Genetic Polymorphism in Healthy Korean Subjects. European Journal of Clinical Pharmacology, 67(9), 889–898. 10.1007/s00228-011-1035-2
- Suzuki, K., Yanagawa, T., Shibasaki, T., Kaniwa, N., Hasegawa, R., & Tohkin, M. (2006). Effect of CYP2C9 Genetic Polymorphisms on the Efficacy and Pharmacokinetics of Glimepiride in Subjects with Type 2 Diabetes. Diabetes Research and Clinical Practice, 72(2), 148–154. 10.1016/j.diabres.2005.09.019
- Douros, A., Yin, H., Yu, O. H. Y., Filion, K. B., Azoulay, L., & Suissa, S. (2017). Pharmacologic Differences of Sulfonylureas and the Risk of Adverse Cardiovascular and Hypoglycemic Events. Diabetes Care, 40(11), 1506–1513. 10.2337/dc17-0595
- Elias, M., & König, M. (2025). A Digital Twin of Glimepiride for Personalized and Stratified Diabetes Treatment. Frontiers in Pharmacology, 16, 1686415. 10.3389/fphar.2025.1686415
- Rosenkranz, B. (1996). Pharmacokinetic Basis for the Safety of Glimepiride in Risk Groups of NIDDM Patients. Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones Et Metabolisme, 28(9), 434–439. 10.1055/s-2007-979833
- Rosenkranz, B., Profozic, V., Metelko, Z., Mrzljak, V., Lange, C., & Malerczyk, V. (1996). Pharmacokinetics and Safety of Glimepiride at Clinically Effective Doses in Diabetic Patients with Renal Impairment. Diabetologia, 39(12), 1617–1624. 10.1007/s001250050624
- Shukla, U. A., Chi, E. M., & Lehr, K.-H. (2004). Glimepiride Pharmacokinetics in Obese versus Non-Obese Diabetic Patients. The Annals of Pharmacotherapy, 38(1), 30–35. 10.1345/aph.1C397
- Grzegorzewski, J., Brandhorst, J., Green, K., Eleftheriadou, D., Duport, Y., Barthorscht, F., Köller, A., Ke, D. Y. J., De Angelis, S., & König, M. (2021). PK-DB: Pharmacokinetics Database for Individualized and Stratified Computational Modeling. Nucleic Acids Research, 49(D1), D1358–D1364. 10.1093/nar/gkaa990
- Hucka, M., Bergmann, F. T., Chaouiya, C., Dräger, A., Hoops, S., Keating, S. M., König, M., Novère, N. L., Myers, C. J., Olivier, B. G., Sahle, S., Schaff, J. C., Sheriff, R., Smith, L. P., Waltemath, D., Wilkinson, D. J., & Zhang, F. (2019). The Systems Biology Markup Language (SBML): Language Specification for Level 3 Version 2 Core Release 2. Journal of Integrative Bioinformatics, 16(2). 10.1515/jib-2019-0021
- Keating, S. M., Waltemath, D., König, M., Zhang, F., Dräger, A., Chaouiya, C., Bergmann, F. T., Finney, A., Gillespie, C. S., Helikar, T., Hoops, S., Malik-Sheriff, R. S., Moodie, S. L., Moraru, I. I., Myers, C. J., Naldi, A., Olivier, B. G., Sahle, S., Schaff, J. C., … SBML Level 3 Community members. (2020). SBML Level 3: An Extensible Format for the Exchange and Reuse of Biological Models. Molecular Systems Biology, 16(8), e9110. 10.15252/msb.20199110
- König, M. (2024). Sbmlutils: Python Utilities for SBML. Zenodo. 10.5281/ZENODO.13325770
- König, M., Dräger, A., & Holzhütter, H.-G. (2012). CySBML: A Cytoscape Plugin for SBML. Bioinformatics, 28(18), 2402–2403. 10.1093/bioinformatics/bts432
- König, M. (2021). Sbmlsim: SBML Simulation Made Easy. 10.5281/ZENODO.5531088





